March 1, 2018
Microscopic revelations point to new blood infection therapies

Thanks to sophisticated imaging techniques and novel “disease-on-chips” technology, researchers at the University of Calgary have for the first time been able to observe — live and in real-time — how the human body responds to often lethal fungal blood infections in the lung. And what they saw surprised them.
Immune cells rushed to the scene of the infection, which was expected. But then those cells swarmed, clustered and jammed up the blood vessels causing a dangerous stroke-like blockage, and that was a revelation, one that could lead to new treatment options for the usually deadly incursions.
The research study which was led by Dr. Bryan Yipp, assistant professor in the Department of Critical Care Medicine at the Cumming School of Medicine and Tier II Canada Research Chair in Pulmonary Immunology, Inflammation and Host Defence, was recently published in CELL Host & Microbe. It grew out of a biomedical engineering project which received Vice-President (Research) matching funds in 2016, in support of the university’s Biomedical Engineering Research Strategy.
Seeking a greater understanding of lung immune response
Yipp says inspiration for the study came from several fronts. He and his team were interested in understanding how the lung in particular is involved in protecting the body from infections. “For some reason the lung itself has a lot of the main type of immune cell, the neutrophil. We knew this, but the reasons why they are in the lung has remained mysterious,” Yipp says.
As well, he says, most research has been on bacterial sepsis but they wanted to focus on fungal sepsis because it is less well understood and fungal infections are growing in number and severity. “Our initial thought was that neutrophils would capture fungi the same as they capture bacteria,” Yipp says. “But they became so activated they started to clump and cluster together and actually block the bloodstream.”
VPR funding focused multidisciplinary expertise
“The big thing the VPR funding allowed us to do was really bring the team together with a bioengineering focus, allowing us to crystallize some ideas about how to approach these complex questions,” Yipp says.
The research group includes Yipp, an ICU doctor, as well as an engineer and several other physician-scientists including Mark Gillrie, Cumming School of Medicine researcher now doing postdoc work at MIT, who developed the study’s high-resolution organs-on-chips.
“I think our results complemented Dr. Yipp’s in vivo work with mouse studies,” Gillrie says. He explains that it’s hard to get good resolution imaging on mouse models, and that also studies in mice don’t always translate into benefits in humans. As a result, the push now is for these organs- and diseases-on-chips that allow scientists to test their hypotheses using human cells.
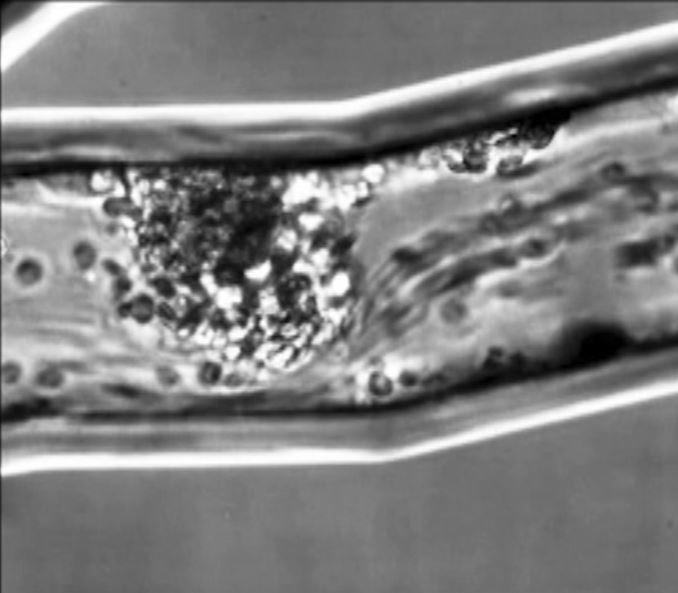
An in vitro model of the lung microvasculature, human neutrophils demonstrate swarming behaviour.
Lee et al. (2018), Movie S11
Advancing diseases-on-chips technology
Chips like the ones intended to mimic lung vasculature are about the size of a penny, with blood vessel structures just microns across. They use the same printing technology as computer chips.
“We generate a pattern of an organ that we want, creating a 3D hollowed-out structure, stick it on a piece of glass, then we can introduce a lot of different cell types,” Gillrie says. Astonishingly, Gillrie says those cells types then have the intrinsic ability to self-organize to a certain degree, into an alveolus in the lung for example, or the blood-brain barrier in the brain.
In the study the fungal infection Candida albicans was introduced to the lung vasculature and as blood was pumped over that system, researchers recorded what happened using highly sophisticated microscopes. That is when they captured in high resolution the clumping of immune cells.
Seeing is believing
“Our real focus is to visualize how everything actually works,” Yipp says, emphasizing the imaging is everything. “These are results we never would have been able to guess at without seeing it.”
With a clearer understanding of the disease — and pictures to prove how the human body reacts to a fungal blood infection in the lung — the researchers say they are looking next to new therapeutic targets.
Yipp says the goal is to find a way to block the clumping of cells without inhibiting the immune cells from capturing bugs. And the team has already had some success with anti-inflammatory drugs already on the market for asthma. “We were able to repurpose a drug that has already been approved in humans that could reduce negative consequences of infection and improve clinical outcomes,” Gillrie says.
The University of Calgary’s multidisciplinary Engineering Solutions for Health: Biomedical Engineering research strategy drives solutions to our most pressing health challenges in disease and injury prevention, diagnosis and treatments. Our biomedical engineering researchers make a significant impact in our communities by extending lives, improving quality of life, promoting independence, and continuously improving the health system.
